Article Text
Abstract
Objective In patients with encephalitis, the development of acute symptomatic seizures is highly variable, but when present is associated with a worse outcome. We aimed to determine the factors associated with seizures in encephalitis and develop a clinical prediction model.
Methods We analysed 203 patients from 24 English hospitals (2005–2008) (Cohort 1). Outcome measures were seizures prior to and during admission, inpatient seizures and status epilepticus. A binary logistic regression risk model was converted to a clinical score and independently validated on an additional 233 patients from 31 UK hospitals (2013–2016) (Cohort 2).
Results In Cohort 1, 121 (60%) patients had a seizure including 103 (51%) with inpatient seizures. Admission Glasgow Coma Scale (GCS) ≤8/15 was predictive of subsequent inpatient seizures (OR (95% CI) 5.55 (2.10 to 14.64), p<0.001), including in those without a history of prior seizures at presentation (OR 6.57 (95% CI 1.37 to 31.5), p=0.025).
A clinical model of overall seizure risk identified admission GCS along with aetiology (autoantibody-associated OR 11.99 (95% CI 2.09 to 68.86) and Herpes simplex virus 3.58 (95% CI 1.06 to 12.12)) (area under receiver operating characteristics curve (AUROC) =0.75 (95% CI 0.701 to 0.848), p<0.001). The same model was externally validated in Cohort 2 (AUROC=0.744 (95% CI 0.677 to 0.811), p<0.001). A clinical scoring system for stratifying inpatient seizure risk by decile demonstrated good discrimination using variables available on admission; age, GCS and fever (AUROC=0.716 (95% CI 0.634 to 0.798), p<0.001) and once probable aetiology established (AUROC=0.761 (95% CI 0.6840.839), p<0.001).
Conclusion Age, GCS, fever and aetiology can effectively stratify acute seizure risk in patients with encephalitis. These findings can support the development of targeted interventions and aid clinical trial design for antiseizure medication prophylaxis.
- AUTOIMMUNE ENCEPHALITIS
- INFECTIOUS DISEASES
- CLINICAL NEUROLOGY
Data availability statement
Data are available upon reasonable request. The de-identified data that support the findings of this study are available from the corresponding author, for any purpose for which there is ethical approval, immediately following publication and ending in September 2022. Researchers should provide a methodologically sound proposal for approval by the UK Health Security Agency, Virus Reference Department. Data are available alongside study protocol.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
We searched MEDLINE for research studies published from 2000 to February 2022, in English, that examined the associations of seizures in encephalitis. Four studies focused on specific clinical settings and subgroups, however, we identified no multicentre studies, and none reported the associations with seizures in encephalitis of all aetiologies.
WHAT THIS STUDY ADDS
This examination of patients with encephalitis reflecting the spectrum of aetiologies from two prospective independent multicentre studies, identified that age, Glasgow Coma Scale on admission, presence of fever and aetiology were strongly associated with seizures. Using these limited parameters in a clinical prediction model, we were able to stratify seizure risk.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings can be used to support the development of targeted interventions, such as early specialist care involvement, for patients at highest risk of seizures and to aid the design of clinical trials of antiseizure medication prophylaxis.
Introduction
Encephalitis is inflammation of the brain parenchyma, caused by infectious or immune-mediated processes, and is associated with significant morbidity and mortality despite antiviral and/or immune therapies.1 Globally, 500 000 children and adults are affected each year.2 The clinical presentation is variable, but typically includes acute or subacute onset of altered mental state alongside fever, headache, new-onset focal neurological signs and, in some, seizures.3 Seizures have particular significance as they are associated with a worse outcome and may well be amenable to prophylaxis.4 Although seizures may be a proxy marker of severe encephalitis, there are a number of mechanisms by which they could lead to further brain inflammation and damage, including hypoxia, excitotoxicity and raised intracranial pressure.5 However, the incidence of acute symptomatic seizures is highly variable (between 2% and 67%).5 Although there is some limited evidence that possible risk factors include the aetiology of encephalitis, younger age and the degree of cortical involvement, our capacity to predict who is at risk of seizures remains very poor.4 5 Consequently, there is insufficient evidence to recommend the use of antiseizure medications (ASM) as standard of care as either primary or secondary prophylaxis.4 6 Initiation and escalation of ASM is possible in most healthcare settings, and if proven to improve outcome, could be started rapidly as ASM therapy is agnostic to eventual aetiology.
Therefore, if a high-risk group were established, this could be used to stratify patients for future clinical trials of primary and secondary prophylaxis with ASM or, as a minimum, to identify which patients should be managed in settings with adequate capacity to manage this severe complication.4 This study aims to establish the factors associated with seizures in encephalitis as well as develop and validate a seizure prediction model of clinical utility for patients presenting with an acute encephalitis syndrome, in accordance with the WHO approach.7 8
Methods
Cohort 1 (development cohort)
Patients were recruited through the Aetiology Study of Encephalitis Study led by the UK Health Protection Agency (now UK Health Security Agency (UKHSA)) (Cohort 1).9 The study prospectively recruited 203 patients with encephalitis from 24 hospitals in England (2005–2008) serving 5 million people (11% of the English population). The study included any person of any age admitted to hospital with encephalitis, full case definition as previously published.3 Computerised tomography (CT), MRI and electroencephalogram (EEG) were performed when clinically indicated. Clinical and postmortem samples received enhanced diagnostic testing guided by a multidisciplinary expert panel.
Cohort 2 (validation cohort)
A second cohort of 233 patients with encephalitis recruited as part of the Understanding and Improving Outcome of Encephalitis in the UK (Enceph-UK) study was used exclusively as a validation cohort for model development (Cohort 2). Enceph-UK prospectively recruited patients from 31 hospitals in England, Wales and Scotland (2013–2016). Patients were eligible if they were 16 years or older and had clinically suspected encephalitis, using the same case definition as Cohort 1.
Statistical analysis
Seizure definition
The three outcome measures were (1) seizure occurrence at any time before or during acute admission, referred to as ‘seizures’, (2) the occurrence of seizures during acute admission, referred to as ‘inpatient seizures’ and (3) the occurrence of status epilepticus. Witness description was used to differentiate focal from generalised seizures. Further subclassification, for example, according to International League Against Epilepsy classification, was not feasible. In Cohort 2, the presence of seizures was recorded as a binary outcome under ‘symptoms on admission (or in current illness, up to 8 weeks prior to admission, including prodrome)’, and, therefore, description of subsequent ‘inpatient’ seizures was not possible.
Data extraction
Data from the first available cerebrospinal fluid (CSF) analysis were extracted. Cut-offs were taken from the UK Standards for Microbiology Investigations: Investigation of CSF.10 Glasgow Coma Scale (GCS) was categorised as normal (15/15), mildly impaired (13–14/15), moderately impaired (9–12/15) or severely impaired (3–8/15). Outcomes were recorded according to the Glasgow Outcome Scale (GOS) 6 months after discharge from hospital. Good recovery was defined as GOS=5 and poor outcome was defined as <5, reflecting at least moderate disability.11
Univariate
All univariate analysis was conducted on Cohort 1. Categorical variables were analysed using χ2 or Fisher’s exact test. All continuous variables that were non-parametric were analysed using Mann-Whitney U test. Potential confounding variables were considered to be age, sex, ethnicity and treatment. These confounders were re-reviewed after univariate analysis and assessed for effect modification and interaction using binary logistic regression.
Model development
Clinical prediction modelling was designed to be applicable to routine clinical practice to stratify seizure risk in patients presenting with the clinical features of acute encephalitis syndrome. Binary logistic regression was used to ascertain predictor variables of seizures in Cohort 1 (SPSS V.26). Due to the limited proportion of patients with a clinically indicated EEG and the risk of data availability bias, EEG results were not considered for inclusion. Collinearity was assessed using correlation matrices and one of any two highly correlated variables omitted. The pattern of missing data was reviewed to assess whether data were missing completely at random. Data not missing ‘completely at random’ by Little’s test but deemed to be missing ‘at random’ were imputed using multiple imputation in clinical variables with >5% missing data. Candidate variables were selected by univariate selection (p<0.25) and those identified in the literature. A selection of strongest contributing predictors was made through backward selection based on likelihood ratio. The final binary logistic regression model based on pooled estimates was converted to a provisional clinical scoring system by dividing regression coefficients of each factor by the smallest regression coefficient among the variables to the nearest integer.
Model development: inpatient seizures
To aid translation to clinical practice, a second binary logistic regression model for inpatient seizure risk was developed using the same approach to represent (1) risk at time point of admission and, (2) with maximal discrimination, and was assessed using receiver operating characteristics (ROC) curve and Hosmer-Lemeshow test (Cohort 1).
Model validation
Both scoring systems were internally validated using leave-one-out cross-validation performed in R (The R Foundation).12 The provisional scoring system for seizures was externally validated on Cohort 2 using ROC curves, Hosmer-Lemeshow test and calibration plot.
Results
Description of Cohort 1
The median (IQR) age was 31 (9–55) years, and 109 (54%) were men. The aetiology included 86 (43%) infectious causes, 42 (21%) immune-mediated and 75 (37%) unknown as previously detailed9 (table 1). Immune-mediated causes included 23 (11%) with acute disseminated encephalomyelitis (ADEM), 9 (4%) N-methyl-D-aspartate receptor antibodies and 7 (3%) were defined as ‘voltage-gated potassium channel’ (VGKC) antibodies. At the time of recruitment, distinction between subtypes of antibody directed at epitopes of the VGKC were not available.
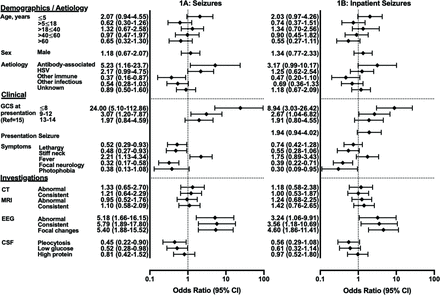
Demographic, clinical and investigatory factors associated with seizures in Cohort 1
Seizures during the acute illness
In Cohort 1, 121 (60%) patients had a seizure during their acute illness and 103 (51%) had a seizure while an inpatient. Of patients with a known presenting report, 43/167 (26%) presented with a history of seizures, which were reported most frequently in patients with autoantibody-associated, 7/14 (50%), and Herpes simplex virus (HSV), 13/29 (45%), aetiologies. A semiotic description of the seizures was available for 73 (36%) patients, of whom 43 (59%) had generalised seizures only, 14 (19%) had focal seizures only and 16 (22%) had both. Four patients had a history of epilepsy, of whom three had a seizure.
Overall, patients with seizures had a lower median (IQR) age at 25 (9–50) years than those without 39 (11–60), (p=0.051) and presented to hospital earlier, at 5 (1–12) versus 9 (4–24) days from symptom onset (p=0.012) (figure 1).
Demographic, clinical and investigatory factors associated with seizures in encephalitis. CSF, cerebrospinal fluid; EEG, electroencephalogram; GCS, Glasgow Coma Scale; HSV, Herpes simplex virus.
Patients with seizures were less likely to have CSF pleocytosis (OR 0.45 (95% CI 0.22 to 0.90), p=0.021) or low CSF glucose (OR 0.52 (95% CI 0.28 to 0.98), p=0.042).
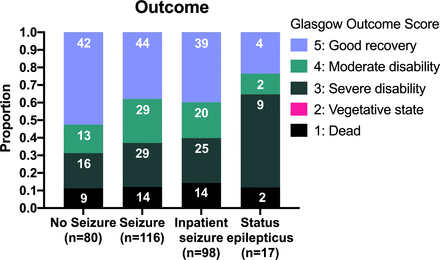
Seizures at any point during the acute illness were associated with a worse outcome, with 42/80 (53%) of those with seizures having a poor outcome as opposed to 44/116 (38%) without seizures (OR 1.81 (95% CI 1.01 to 3.23), p=0.044) (figure 2).
Glasgow Outcome Scale at 6 months stratified by presence and nature of seizures.
Inpatient seizures
In Cohort 1, inpatient seizures were present in 27/43 (67%) of patients presenting with a history of seizures, and 64/124 (52%) without a history of seizures. In patients who did not present with a history of seizures, those that would go on to develop an inpatient seizure presented with a shorter duration of symptoms at 6 (1–13) versus 9 (4–21) days (p=0.034).
Reduced GCS on admission was associated with subsequent inpatient seizures. This association remained when stratifying data according to whether the patient presented with a history of seizures. In 92 patients with known GCS and without a history of seizures at presentation, those with severely impaired GCS were more likely to have at least one subsequent inpatient seizure (11/13 (85%), OR 6.57 (95% CI 1.37 to 31.5)), compared with those with moderately impaired (11/21 (52%), OR 1.07 (95% CI 0.40 to 2.93)) or mildly impaired/normal GCS (25/58 (43%), OR 0.41 (95% CI 0.17 to 0.99)) (p=0.025).
Status epilepticus
Status epilepticus occurred in 19/203 (9%) of patients with median (IQR) age of 20 (6–31) years. Three had non-convulsive status epilepticus (NCSE) identified on EEG. Four patients had autoantibody-associated encephalitis, three HSV, two Mycobacterium tuberculosis, one probable influenza A, one ADEM and eight had an unknown aetiology. Patients with status epilepticus were more likely to present with a seizure, 10/19 (53%) (of whom three presented with status epilepticus), than those who did not develop status epilepticus 33/148 (22%) (p=0.004). Fever was present in all patients with status epilepticus (19/19, 100%, p=0.009).
All 17 EEGs were abnormal, 16/17 (94%) were consistent with encephalitis and 15/17 (88%) had focal changes. These focal changes were significantly more frequently identified in patients with status epilepticus, 15/17 (88%), compared with those without status epilepticus, 31/92 (34%) (p<0.001).
The probability of subsequent disability was significantly higher in patients with status epilepticus. In survivors with previous status epilepticus a minority, 4/15 (26%) made a good recovery, 2/15 (13%) had mild disability and most 9/15 (60%) had severe disability (figure 2). In survivors without history of status epilepticus most, 82/158 (52%), made a good recovery, 40/158 (25%) had mild disability and 36/158 (23%) severe disability (p=0.028).
Description of Cohort 2
Patients in Cohort 2 were older, median (IQR), 54 (34–68) years, more likely to be of white ethnicity, 210/233 (91%), and less frequently reported to have a history of seizures, 84/233 (36%), or fever, 102/233 (44%) (online supplemental table 1). Consistent with Cohort 1, autoimmune and HSV aetiology (p=0.002) and low GCS on admission (p<0.001) were associated with seizures.
Supplemental material
Provisional model
Presenting with a seizure and GCS were co-linear, however, GCS was most strongly associated with seizures and likely also captures whether a patient has a history of seizures due to the postictal phase, so was retained in the model. The provisional model of seizures at any time included GCS on admission and probable aetiology of encephalitis (χ2=42.53, p<0.001) (online supplemental table 2). Consistent with the univariate analysis, autoantibody-associated (OR 11.99 (95% CI 2.09 to 68.86), p=0.017) and HSV encephalitis (3.58 (95% CI 1.06 to 12.12), p=0.096) were associated with seizures. Internal cross-validation demonstrated 68% sensitivity, 72% specificity, with a positive predictive value (PPV) of 62% and negative predictive value (NPV) of 77%, with overall accuracy 70%.
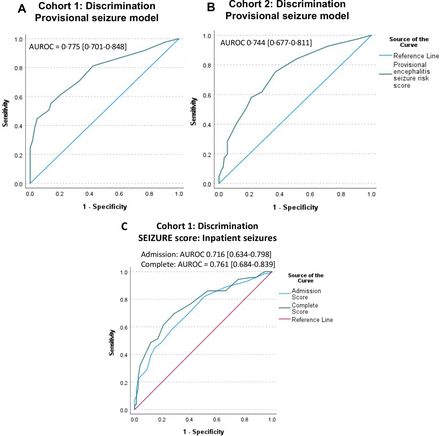
The model demonstrated good discrimination in Cohort 1 and when externally validated in Cohort 2, area under ROC (AUROC)=0.775 (95% CI 0.701 to 0.848) and 0.744 (95% CI 0.677 to 0.811) respectively (figure 3) and Hosmer-Lemeshow test equalled p=0.737 on the original data. Further evaluation of provisional model calibration is provided in online supplemental table 3 and online supplemental figure 1. The provisional model systematically overestimated risk in Cohort 2, but seizures were less commonly reported in Cohort 2 compared with Cohort 1, 84/233 (36%) and 121/203 (60%) patients, respectively.
Model performance. Receiver operating characteristics (ROC) curve for seizure risk according to provisional seizure model in Cohort 1 (A), Area under ROC (AUROC)=0.775 (95% CI 0.701 to 0.848), and Cohort 2 (B), AUROC 0.744 (95% CI 0.6770.811). (C) ROC curve for inpatient seizure risk according to SEIZUre Risk in Encephalitis (SEIZURE) score in Cohort 1.
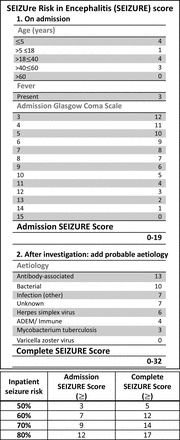
Inpatient seizure risk: SEIZURE score
A second binary logistic regression model was developed to identify predictors of inpatient seizures based on the information available on admission and then on these parameters combined with aetiology once established (Cohort 1) (table 2). The derived, points-based SEIZUre Risk in Encephalitis (SEIZURE) score stratified risk by decile and is designed to be applied by healthcare professionals when a patient of any age with suspected encephalitis is admitted to hospital with two weighted scoring systems for application prior to and following identification of the probable aetiology (figure 4). Internal cross-validation demonstrated 66% sensitivity, 72% specificity, PPV 69% and NPV 69%, with overall accuracy 69%. Patients in the highest risk category on admission had an OR 7.17 (95% CI 2.55 to 20.16) of seizures compared with those in the lowest risk categories and an OR of 15.51 (95% CI 5.60 to 42.96) once probable aetiology was established (table 3).
SEIZUre Risk in Encephalitis (SEIZURE) score for stratifying inpatient seizure risk by decile. ADEM, acute disseminated encephalomyelitis.
Development of scoring system for inpatient seizure risk in encephalitis using binary logistic regression model based on pooled estimates from imputed data in Cohort 1
Performance of SEIZURE score by cut-off in complete data, Cohort 1
The admission SEIZURE score, including age, admission GCS and fever, showed good discrimination (AUROC=0.716 (95% CI 0.634 to 0.798)). Addition of probable aetiology, once known, slightly increased discrimination (AUROC 0.761 (95% CI 0.684 to 0.839), p<0.001) and Hosmer-Lemeshow for the complete model was p=0.285 on original data.
Discussion
Acute seizures affect many patients with encephalitis and are associated with increased need for intensive care support and a worse outcome, and moreover may further contribute to brain injury through excitotoxicity, host immune responses and raised intracranial pressure.5 12–15 However, there are currently no established tools to stratify patients as to their risk of seizures and status epilepticus. Without such risk stratification, it is currently not possible to identify which patients would be best managed in centres with adequate clinical facilities, such as those with intensive therapy units and continuous EEG monitoring, and also to identify whom might benefit from primary and secondary ASM prophylaxis.
Through our evaluation of two independent prospective multicentre cohort studies, we identified multiple factors associated with increased risk of seizures during the acute illness, particularly low GCS on admission, fever and an autoantibody-associated or HSV aetiology. Patients with seizures presented to hospital earlier, even in those who have not yet had their first seizure at the time of presentation. Models for seizure risk could be established which, despite requiring a small number of variables, were strongly predictive of acute seizures. Low GCS on admission was more strongly associated with inpatient seizures than whether the patient presented with a seizure history or not. The provisional score accurately determined seizure risk in the first cohort, but potentially overestimated seizure risk in the second cohort, perhaps reflecting under-documentation of seizures in this cohort as seizures were limited to those documented at presentation. The ‘SEIZURE score’ for inpatient seizures requires further external validation. Improved access to easy EEG-monitoring on a wider scale, or the establishment of novel biomarkers could enhance the accuracy of risk-stratification. In addition, the impact of ASM prescription as primary and secondary prophylaxis requires further evaluation.4 5
There are likely to be multiple structural and biochemical mechanisms underlying seizure risk in patients with acute encephalitis syndrome. For example, HSV encephalitis has a predisposition to affect epileptogenic areas within the frontotemporal region and in autoimmune encephalitis, the antibodies associated with neuronal cell-surface antigens, that are highly expressed in this region, are themselves often directly involved in the disease process.16–18 The differential disease mechanisms observed in specific aetiologies of encephalitis may influence seizure risk, however, there was inadequate power in this analysis to establish factors associated with seizures within aetiological subgroups. Certain clinical features were less common in patients with seizures, specifically, stiff neck, photophobia, lethargy and any focal deficit on neurological examination. This may in part be explained by aetiological distinctions, as focal neurological deficits were most frequently reported in encephalitis caused by Varicella zoster virus and ADEM, which were least strongly associated with seizures; and particularly the latter which is associated with subcortical white matter lesions as opposed to cortical inflammation which drives seizures.19 Many clinical features associated with seizures in our analysis are likely to be proxies for underlying mechanisms, rather than risk factors in themselves. Nevertheless, these features can inform future mechanistic studies, particularly through in vivo models of encephalitis.20
EEG abnormalities were strongly associated with clinical seizure activity and EEG also identified three cases of NCSE, which is increasingly recognised in encephalitis, particularly autoimmune aetiologies.21 22 Status epilepticus in the context of encephalitis is frequently refractory and has a poor outcome.23 24 Patients with seizures were less likely to have CSF pleocytosis or low CSF glucose. Given that lumbar puncture is contraindicated until patients are stabilised following a seizure, we hypothesise that this result could be related in part to delayed lumbar puncture, or also may reflect the increased proportion with autoimmune encephalitis in this group.19 These CSF parameters likely reflect aetiological distinctions rather than direct biomarkers of seizures. Nevertheless, these data sets did not provide sufficient granularity to determine the CSF white cell count relative to the time from/before a seizure and this requires further study. No relationship was observed between the presence of normal or abnormal CT or MRI findings and seizure risk. It may be that the imaging variables in this analysis were too crude as they were based on retrospective interpretation of clinically indicated scans performed at multiple sites. Volumetric analysis for research purposes of specific brain regions or structures would be more sensitive.6 25–27 The finding may additionally reflect the high incidence of seizures in those with autoimmune encephalitis, who often have normal or near-normal neuroimaging. A single-centre study of 94 patients in China found cortical or hippocampal abnormalities on neuroimaging independently predicted progression to super-refractory status epilepticus.28 Notably, the potential associations of seizures identified in our study; aetiology, GCS and younger age, were also reported in a single-centre study in Northern India, despite large differences in the cohort.26 The likelihood of a seizure being witnessed may confound associations, for example, younger children in community settings may be more likely to have a seizure witnessed.
Our study corroborates previous work demonstrating an association between seizure activity and poor outcome in encephalitis.14 29–31 Although seizures may be a proxy marker of severe disease, there are a number of mechanisms through which seizures could cause further brain damage. Seizures cause significant systemic metabolic and biochemical disturbance including hypoxia, hypoglycaemia, metabolic acidosis as well as direct central nervous system perturbations including glutaminergic activity, raised intracranial pressure and blood–brain barrier permeabilisation, as well as low CSF glucose and high CSF lactate.32 A study of 144 patients with Japanese encephalitis presenting to hospital in Vietnam, showed that patients with recent seizures had high CSF lactate:glucose ratios and high CSF opening pressures and that patients with opening pressure >25 cm were more likely to die.14 A more recent analysis of CSF biomarkers in HSV encephalitis indicated that acute inflammation may drive subsequent synaptic autoimmunity and proposed a trial of post-acute corticosteroids.33 In addition, several inflammatory markers have been associated with a lower GCS, increased oedema and a worse outcome in encephalitis, especially the interleukin-1 family relative to their endogenous antagonists.13 It remains unknown whether seizures intervene with the underlying encephalitic process.
Despite the high prevalence and prognostic importance of seizures, the most recent Cochrane review concluded that there is insufficient evidence to support or refute the routine use of antiepileptic drugs for the primary or secondary prevention of seizures in viral encephalitis.4 A recent randomised controlled trial of secondary prophylaxis for acute symptomatic seizures in children with encephalitis demonstrated that a 4-week course of ASM was comparable to 12 weeks in terms of the incidence of seizure recurrence.34 A rabbit model of HSV-1 encephalitis, untreated with aciclovir, showed that all animals that had a seizure became moribund or died, but that phenobarbital prevented seizures and significantly reduced mortality.35 Further questions remain regarding choice and duration of antiepileptic agents.6 Any intervention strategy would need to consider the high baseline risk of seizures in patients with encephalitis and the presence of subtle and subclinical seizures including NCSE.14 21 36
Our findings reflect two relatively large prospectively recruited cohorts but have limitations, principally due to the retrospective nature of seizure-focused analysis. The observational nature of the study has an intrinsic risk of confounding which we have attempted to address in both the analysis and interpretation of results but there may be a residual impact. Additionally, the data were collected from 2005 to 2016 and seizures were not the primary focus of the initial data collection, potentially resulting in missing data. The diagnosis and management of encephalitis may have changed over this time period, particularly the identification of autoantibodies. These factors are balanced by the substantial sample size for a relatively uncommon condition, the granularity of the UKHSA data and the enhanced diagnostic testing performed.
Conclusion
These finding indicate that patients with seizures during encephalitis present earlier, but despite this, have a worse outcome, suggesting there may be a window of opportunity for intervention that is currently not being exploited. This study provides a foundation for risk stratification of seizures in encephalitis on clinical grounds alone. Biomarkers and improved access to EEG-monitoring could enhance model accuracy and allow for the development of targeted interventions. The SEIZURE score can be used to aid the design of clinical trials of primary and secondary prophylaxis with ASM.
Data availability statement
Data are available upon reasonable request. The de-identified data that support the findings of this study are available from the corresponding author, for any purpose for which there is ethical approval, immediately following publication and ending in September 2022. Researchers should provide a methodologically sound proposal for approval by the UK Health Security Agency, Virus Reference Department. Data are available alongside study protocol.
Ethics statements
Patient consent for publication
Ethics approval
The original HPA study had ethical approval granted by The North and East Devon Multicentre Research Ethics Committee (05/Q2102/22). The proposal for this follow-on study was granted approval by Public Health England (now UK Health Security Agency). The ENCEPH-UK Study was approved by the East Midlands Committee of the National Research Ethics Service (NRES) (11/EM/0442). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We would like to thank the patients involved in this research. UK Health Protection Agency Aetiology of Encephalitis Study Group: Helen E Ambrose, Nicholas W S Davies, Jonathan P Clewley, Amanda L Walsh, Dilys Morgan, Richard Cunningham, Mark Zuckerman, Ken J Mutton, Katherine N Ward, Michael P T Lunn, Natasha S Crowcroft, Craig Ford, Emily Rothwell, William Tong, Jean-Pierre Lin, Ming Lim, Nicholas Price, Javeed Ahmed, David Cubitt, Sarah Benton, Cheryl Hemingway, David Muir, Hermione Lyall, Ed Thompson, Geoff Keir, Viki Worthington, Paul Griffiths, Susan Bennett, Rachel Kneen, Paul Klapper. ENCEPH-UK Study Group: Ruth Backman, Gus Baker, Nicholas J Beeching, Rachel Breen, Chris Cheyne, Enitan D Carrol, Nicholas W S Davies, Martin Eccles, Robbie Foy, Marta Garcia-Finana, Julia Griem, Michael Griffiths, Alison Gummery, Lara Harris, Helen Hickey, Helen Hill, Ann Jacoby, Hayley Hardwick, Ciara Kierans, Michael Kopelman, Rachel Kneen, Gill Lancaster, Michael Levin, Rebecca McDonald, Antonieta Medina-Lara, Esse Menson, Natalie Martin, Andrew Pennington, Andrew Pollard, Julie Riley, Manish Sadarangani, Anne Salter, Maria Thornton, Charles Warlow. AGM is a National Institute for Health Research (NIHR) Senior Investigator and also part funded by NIHR ARC North West Coast.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @gkwood3
Contributors JG, TS and DWGB initiated the original HPA study, obtained funding and recruited hospitals to the study. JG led HPA study design. GKW and BDM designed secondary analysis. GKW did the literature search. GKW, BDM, KT and GB did the statistical analysis. GKW and BDM developed the figures. GKW, TS and BDM wrote the first draft. BDM is the guarantor of the paper. All authors contributed to interpretation of data and critically approved the final paper.
Funding Funded by the Department of Health Policy Research Programme (Enhanced Diagnostic and Management Strategies to Improve the Identification and Outcome of Individuals with Encephalitis), 047/1084. BDM is supported by the UKRI/MRC (MR/V03605X/1), the MRC/UKRI (MR/V007181//1), MRC (MR/T028750/1) and Wellcome (ISSF201902/3).
Disclaimer The sponsors of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests RHT has received honoraria and meeting support from Arvelle, Bial, Eisai, GW Pharma, LivaNova, Novartis, Sanofi, UCB Pharma, UNEEG, Zogenix. Conflicts of Interest. SRI is an inventor on ‘Diagnostic Strategy to improve specificity of CASPR2 antibody detection (PCT/G82019 /051257) and receives royalties on a licenced patent application for LGI1/CASPR2 testing as coapplicant (PCT/GB2009/051441) entitled ‘Neurological Autoimmune Disorders’. AV and University of Oxford hold a patent for VGKC-complex (LGI1 and CASPR2) antibody tests, licenced to Euroimmune AG. AV receives a proportion of royalties. SRI is supported by a senior clinical fellowship from the Medical Research Council (MR/V007173/1), Wellcome Trust Fellowship (104079/Z/14/Z) and the BMA Research Grants - Vera Down grant (2013) and Margaret Temple (2017), Epilepsy Research UK (P1201), the Fulbright UK-US commission (MS-Society research award) and by the NIHR Oxford Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. SRI has received honoraria from UCB, Immunovant, MedImmun, Roche, Cerebral therapeutics, ADC therapeutics, Brain, Medlink Neurology and research support from CSL Behring, UCB and ONO Pharma. TS was Chair/Co-Chair of the UKRI/NIHR COVID-19 Rapid Response and Rolling Funding Initiatives (from March 2020), an Advisor to the UK COVID-19 Therapeutics Advisory Panel (UK-TAP, from August 2020) and a member of the MHRA COVID-19 Vaccines Benefit Risk Expert Working Group (from August 2020). He is also a trustee of the UK Academy of Medical Sciences (December 2021). TS is President of the Encephalitis Society. TS was an advisor to the GSK Ebola Vaccine programme and chaired a Siemens Diagnostics Clinical Advisory Board. TS was on the Data Safety Monitoring Committee of the GSK Study to Evaluate the Safety and Immunogenicity of a Candidate Ebola Vaccine in Children GSK3390107A (ChAd3 EBO-Z) vaccine. TS chaired the Siemens Healthineers Clinical Advisory Board. Data safety monitoring board: Study of Ebola vaccine and ChAd3-EBO-Z - Commercial entity. BDM is supported by the UKRI/MRC (MR/V03605X/1), the MRC/UKRI (MR/V007181//1), MRC (MR/T028750/1) and Wellcome (ISSF201902/3).
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.