Abstract
Purpose
Radiological hallmark of autoimmune limbic encephalitis (LE) is a hyperintense signal in MRI T2-weighted images of mesial temporal structures. We aimed to identify conventional magnetic resonance imaging (MRI) features that can help distinguish LE from temporal glioma.
Methods
Brain MRIs of 25 patients affected by antibody-positive autoimmune LE, 24 patients affected by temporal glioma (tumor group), and 5 negative controls were retrospectively blindly evaluated in random order.
Results
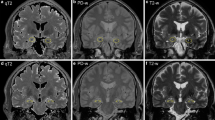
Ten brain MRIs from the LE group were correctly recognized; one additional patient with mesial temporal hyperintensity with anti-AK5 abs LE was wrongly diagnosed as having a tumor. The brain MRIs of the remaining 14 of the 25 patients with LE were judged negative or, in three cases, showed features not typical for LE. In the tumor group, all MRIs showed pathological alterations diagnosed as tumors in 22/24 cases and as LE in two (2/22, 9%). Unilateral lesions were more common in tumors than in neuroradiologically abnormal LE (96% vs. 18%, p < 0.001). T2/FLAIR hyperintensity of the parahippocampal gyrus was associated more with tumor than with LE (71% vs. 18%) (p = 0,009), as T2/FLAIR hyperintensity of extralimbic structures (p = 0.015), edema (p = 0.041), and mass effect (p = 0.015). Maintenance of gray/white matter distinction was strongly associated with LE (91% vs. 17%, p < 0.001).
Conclusion
Conventional brain MRI is a fundamental tool in the differential diagnosis between LE and glioma. Bilateral involvement and maintenance of gray/white matter distinction at the cortical/subcortical interface are highly suggestive of LE.




Similar content being viewed by others
References
Demaerel P, Van Dessel W, Van Paesschen W et al (2011) Autoimmune-mediated encephalitis. Neuroradiology 53:837–851. https://doi.org/10.1007/s00234-010-0832-0
Graus F, Titulaer MJ, Balu R, et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15:391–404. https://doi.org/10.1016/S1474-4422(15)00401-9
Vogrig A, Joubert B, Ducray F, Thomas L, Izquierdo C, Decaestecker K, Martinaud O, Gerardin E, Grand S, Honnorat J (2018) Glioblastoma as differential diagnosis of autoimmune encephalitis. J Neurol 265:669–677. https://doi.org/10.1007/s00415-018-8767-1
Hart IK, Waters C, Vincent A, Newland C, Beeson D, Pongs O, Morris C, Newsom-Davis J (1997) Autoantibodies detected to expressed K+ channels are implicated in neuromyotonia. Ann Neurol 41:238–246. https://doi.org/10.1002/ana.410410215
Saiz A, Blanco Y, Sabater L, González F, Bataller L, Casamitjana R, Ramió-Torrentà L, Graus F (2008) Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain 131:2553–2563. https://doi.org/10.1093/brain/awn183
Bernal F, Shams’ili S, Rojas I et al (2003) Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 60:230–234. https://doi.org/10.1212/01.WNL.0000041495.87539.98
van Sonderen A, Schreurs MWJ, de Bruijn MAAM, Boukhrissi S, Nagtzaam MMP, Hulsenboom ESP, Enting RH, Thijs RD, Wirtz PW, Sillevis Smitt PAE, Titulaer MJ (2016) The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology 86:1692–1699. https://doi.org/10.1212/WNL.0000000000002637
Louis DN, Ohgaki H, Wiestler ODCW (2016) World Health Organization histological classification of tumours of the central nervous system. International Agency for Research on Cancer, France
Athauda D, Delamont RS, De P-FE (2014) High grade glioma mimicking voltage gated potassium channel complex associated antibody limbic encephalitis. Case Rep Neurol Med 2014:1–4. https://doi.org/10.1155/2014/458790
Cope TE, Breen DP, Chawda S, Cifelli A (2016) Anti-collapsin response mediator protein 5 encephalitis masquerading as a low-grade brain tumour. Pract Neurol 16:376–380. https://doi.org/10.1136/practneurol-2016-001379
Deramecourt V, Bombois S, Debette S, Delbeuck X, Ramirez C, Reyns N, Kerdraon O, Maurage CA, Pasquier F (2009) Bilateral temporal glioma presenting as a paraneoplastic limbic encephalitis with pure cognitive impairment. Neurologist. 15:208–211. https://doi.org/10.1097/NRL.0b013e31818fc022
Kerling F, Blümcke I, Stefan H (2008) Pitfalls in diagnosing limbic encephalitis—a case report. Acta Neurol Scand 118:339–342
Nagata R, Ikeda K, Nakamura Y, Ishikawa Y, Miura K, Sato R, Kawase Y, Kawabe K, Iwasaki Y (2010) A case of Gliomatosis Cerebri mimicking limbic encephalitis: malignant transformation to glioblastoma. Intern Med 49:1307–1310
Simo M, Dalmau J (2009) Cerebral gliomatosis simulating limbic encephalitis. Neurologia 24:500–501
Schulz UG, Stewart W, Thomas SR (2009) A difficult case solved at autopsy: memory loss, behavioural change and seizures. Pract Neurol 9:90–95
Urbach H, Soeder BM, Jeub M, Klockgether T, Meyer B, Bien CG (2006) Serial MRI of limbic encephalitis. Neuroradiology 48:380–386. https://doi.org/10.1007/s00234-006-0069-0
Oyanguren B, Sánchez V, González FJ, de Felipe A, Esteban L, López-Sendón JL, Garcia-Barragán N, Martínez-San Millán J, Masjuán J, Corral I (2013) Limbic encephalitis: a clinical-radiological comparison between herpetic and autoimmune etiologies. Eur J Neurol 20:1566–1570. https://doi.org/10.1111/ene.12249
Kotsenas AL, Watson RE, Pittock SJ, Britton JW, Hoye SL, Quek AML, Shin C, Klein CJ (2014) MRI findings in autoimmune voltage-gated potassium channel complex encephalitis with seizures: one potential etiology for mesial temporal sclerosis. Am J Neuroradiol 35:84–89. https://doi.org/10.3174/ajnr.A3633
Chourmouzi D, Papadopoulou E, Marias K, Drevelegas A (2014) Imaging of brain tumors. Surg Oncol Clin N Am 23:629–684. https://doi.org/10.1016/j.soc.2014.07.004
Dean BL, Drayer BP, Bird CR, Flom RA, Hodak JA, Coons SW, Carey RG (1990) Gliomas: classification with MR imaging. Radiology 174:411–415. https://doi.org/10.1148/radiology.174.2.2153310
Ng ASL, Kramer J, Centurion A, Dalmau J, Huang E, Cotter JA, Geschwind MD (2015) Clinico-pathological correlation in adenylate kinase 5 autoimmune limbic encephalitis. J Neuroimmunol 287:31–35. https://doi.org/10.1016/j.jneuroim.2015.08.009
Tüzün E, Rossi JE, Karner SF, Centurion AF, Dalmau J (2007) Adenylate kinase 5 autoimmunity in treatment refractory limbic encephalitis. J Neuroimmunol 186:177–180. https://doi.org/10.1016/j.jneuroim.2007.03.015
Do LD, Chanson E, Desestret V, Joubert B, Ducray F, Brugière S, Couté Y, Formaglio M, Rogemond V, Thomas-Antérion C, Borrega L, Laurens B, Tison F, Curot J, de Brouker T, Lebrun-Frenay C, Delattre JY, Antoine JC, Honnorat J (2017) Characteristics in limbic encephalitis with anti-adenylate kinase 5 autoantibodies. Neurology 88:514–524. https://doi.org/10.1212/WNL.0000000000003586
Flanagan EP, Kotsenas AL, Britton JW, McKeon A, Watson RE, Klein CJ, Boeve BF, Lowe V, Ahlskog JE, Shin C, Boes CJ, Crum BA, Laughlin RS, Pittock SJ (2015) Basal ganglia T1 hyperintensity in LGI1-autoantibody faciobrachial dystonic seizures. Neurology 2. https://doi.org/10.1212/NXI.0000000000000161
Tripathi M, Tripathi M, Roy SG, Parida GK, Ihtisham K, Dash D, Damle N, Shamim SA, Bal C (2018) Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology 60:189–198. https://doi.org/10.1007/s00234-017-1956-2
Urbach H, Rauer S, Mader I, Paus S, Wagner J, Malter MP, Prüss H, Lewerenz J, Kassubek J, Hegen H, Auer M, Deisenhammer F, Ufer F, Bien CG, Baumgartner A (2015) Supratentorial white matter blurring associated with voltage-gated potassium channel-complex limbic encephalitis. Neuroradiology 57:1203–1209. https://doi.org/10.1007/s00234-015-1581-x
Kim JA, Chung J, Ho Yoon P et al (2001) Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: Periictal diffusion-weighted imaging. Am J Neuroradiol 22:1149–1160
Gultekin SH, Rosenfeld MR, Voltz R et al (2000) Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 123(Pt 7):1481–1494
van Sonderen A, Thijs RD, Coenders EC, Jiskoot LC, Sanchez E, de Bruijn MAAM, van Coevorden-Hameete MH, Wirtz PW, Schreurs MWJ, Sillevis Smitt PAE, Titulaer MJ (2016) Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 87:1449–1456. https://doi.org/10.1212/WNL.0000000000003173
Finke C, Prüss H, Heine J, Reuter S, Kopp UA, Wegner F, Then Bergh F, Koch S, Jansen O, Münte T, Deuschl G, Ruprecht K, Stöcker W, Wandinger KP, Paul F, Bartsch T (2017) Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol 74:50–59. https://doi.org/10.1001/jamaneurol.2016.4226
van Sonderen A, Ariño H, Petit-Pedrol M, Leypoldt F, Körtvélyessy P, Wandinger KP, Lancaster E, Wirtz PW, Schreurs MWJ, Sillevis Smitt PAE, Graus F, Dalmau J, Titulaer MJ (2016) The clinical spectrum of Caspr2 antibody-associated disease. Neurology 87:521–528. https://doi.org/10.1212/WNL.0000000000002917
Hirai T, Korogi Y, Yoshizumi K, Shigematsu Y, Sugahara T, Takahashi M (2000) Limbic lobe of the human brain: evaluation with turbo fluid-attenuated inversion-recovery MR imaging. Radiology 215:470–475. https://doi.org/10.1148/radiology.215.2.r00ma06470
Coan AC, Kubota B, Bergo FPG, Campos BM, Cendes F (2014) 3T MRI quantification of hippocampal volume and signal in mesial temporal lobe epilepsy improves detection of hippocampal sclerosis. Am J Neuroradiol 35:77–83. https://doi.org/10.3174/ajnr.A3640
Wagner J, Schoene-Bake JC, Malter MP, Urbach H, Huppertz HJ, Elger CE, Weber B (2013) Quantitative FLAIR analysis indicates predominant affection of the amygdala in antibody-associated limbic encephalitis. Epilepsia 54:1679–1687. https://doi.org/10.1111/epi.12320
Acknowledgments
The authors thank Francesc Graus (Barcelona, Spain) for providing cell-based assay for AK5 antibody and Angela Vincent (Oxford, UK) for VGKC radioimmunoassay, and Joanne Fleming for reviewing the manuscript.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures performed in the studies involving human participants were in accordance with the ethical standards of our institutional research committees and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zoccarato, M., Valeggia, S., Zuliani, L. et al. Conventional brain MRI features distinguishing limbic encephalitis from mesial temporal glioma. Neuroradiology 61, 853–860 (2019). https://doi.org/10.1007/s00234-019-02212-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02212-1